Pfizer and Moderna Trial Data Shows a Higher Risk of Serious Adverse Events Among Vaccinated Participants, a New Study Finds
by Rhoda Wilson
A peer-reviewed study published two days ago found that both the Moderna and the Pfizer vaccine trial data appeared to have a negative benefit/harm ratio. One of the authors of the paper is Peter Doshi, an associate editor of the British Medical Journal.
Using a prespecified list of adverse events of special interest (“AESI”) identified by the Brighton Collaboration, the study found a higher risk of serious AESI in the mRNA COVID-19 vaccine group relative to placebo in both the Pfizer and Moderna adult phase III trials.
“These results raise concerns that mRNA vaccines are associated with more harm than initially estimated at the time of emergency authorisation,” the study authors wrote.
Adverse Events of Special Interest (“AESI”)
In 2020, before the mass Covid injection campaign, the Brighton Collaboration created a priority list of Covid injection AESIs. This list was endorsed by the World Health Organisation. “To our knowledge, however, the list has not been applied to serious adverse events in randomised trial data,” the study authors wrote.
So, the researchers adapted this list to evaluate AESIs observed in both the Pfizer and Moderna mRNA Covid-19 vaccine phase III randomised, placebo-controlled clinical trials on which the US Food and Drug Administration (“FDA”) emergency use authorisation was based:
We conducted a simple comparison of harms with benefits to illustrate the need for formal harm-benefit analyses of the vaccines that are stratified according to risk of serious Covid-19 outcomes. Our analysis is restricted to the randomised trial data, and does not consider data on post-authorisation vaccination program impact.
Joseph Fraiman, Juan Erviti, Mark Jones, Sander Greenland, Patrick Whelan, Robert M. Kaplan, Peter Doshi, Serious adverse events of special interest following mRNA COVID-19 vaccination in randomised trials in adults, Vaccine, 2022, ISSN 0264-410X, https://doi.org/10.1016/j.vaccine.2022.08.036.
The authors found:
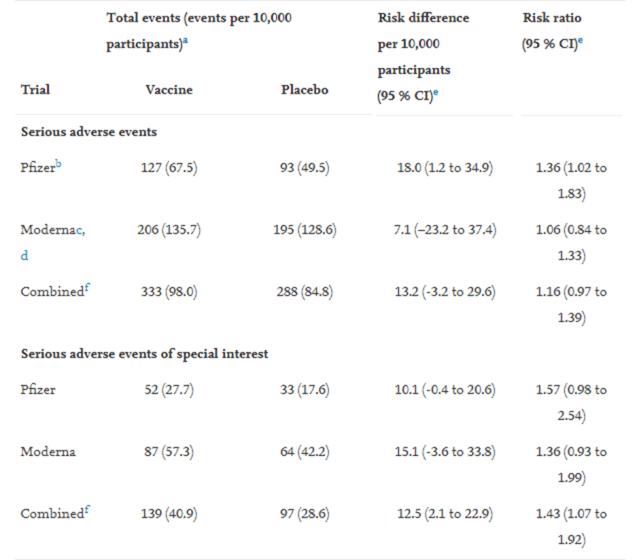
a 57 % higher risk of serious AESI in vaccinated trial participants (Pfizer);
a 36 % higher risk of serious AESI in vaccinated trial participants (Moderna);
combining the trials, there was a 43 % higher risk of serious AESI in vaccinated participants; and,
in both Pfizer and Moderna trials, the largest excess risk occurred in the category of coagulation disorders.
The authors also noted that “Cardiac disorders have been of central concern for mRNA vaccines; in the Pfizer trial more cardiovascular AESIs occurred in the vaccine group than in the placebo group, but in the Moderna trial the groups differed by only 1 case.”
Other key findings from the paper:
In the Moderna trial, the excess risk of serious AESIs (15.1 per 10,000 participants) was higher than the risk reduction for Covid hospitalisation relative to the placebo group (6.4 per 10,000 participants).
In the Pfizer trial, the excess risk of serious AESIs (10.1 per 10,000) was higher than the risk reduction for Covid hospitalisation relative to the placebo group (2.3 per 10,000 participants).
Serious Adverse Events (“SAEs”)
The study noted that SAEs were defined in the trial protocols and supplemental material. Accordingly, “an SAE was defined as an adverse event that results in any of the following conditions: death; life-threatening at the time of the event; inpatient hospitalisation or prolongation of existing hospitalisation; persistent or significant disability/incapacity; a congenital anomaly/birth defect; medically important event, based on medical judgment.”
The study found that the Pfizer trial exhibited a 36 % higher risk and the Moderna trial exhibited a 6 % higher risk of SAEs in the vaccine group. Combined, there was a 16 % higher risk of SAEs in mRNA vaccine recipients.
Comparing their findings with the FDA’s review, the study authors noted:
In their review of SAEs supporting the authorisation of the Pfizer and Moderna vaccines, the FDA concluded that SAEs were, for Pfizer, “balanced between treatment groups,” and for Moderna, were “without meaningful imbalances between study arms.” In contrast to the FDA analysis, we found an excess risk of SAEs in the Pfizer trial. Our analysis of Moderna was compatible with FDA’s analysis, finding no meaningful SAE imbalance between groups.
The table below summarises the study’s findings.
Limitations
Under the discussion section, beginning in the fourth paragraph, the authors noted the limitations of their study, including:
Pfizer’s trial did not report SAEs occurring past 1 month after dose 2. This reporting threshold may have led to an undercounting of serious AESIs in the Pfizer trial.
For both studies, the limited follow-up time prevented an analysis of harm-benefit over a longer period.
All SAEs in their analysis met the regulatory definition of a serious adverse event, but many adverse event types that a patient may themselves judge as serious may not meet this regulatory threshold.
Decisions about which SAEs to include or exclude as AESIs require subjective, clinical judgements in the absence of detailed clinical information about the actual SAEs.
A lack of access to individual participant data, which forced them to use a conservative adjustment to the standard errors, “The 95 % CIs calculated are therefore only approximate because we do not know which patients had multiple events.”
In the Moderna analysis, the SAEs that were sequelae of serious Covid-19 could not be identified and therefore remain included in their calculations.
We emphasise that our investigation is preliminary, to point to the need for more involved analysis. The risks of serious AESIs in the trials represent only group averages. SAEs are unlikely to be distributed equally across the demographic subgroups enrolled in the trial, and the risks may be substantially less in some groups compared to others. Thus, knowing the actual demographics of those who experienced an increase in serious AESI in the vaccine group is necessary for a proper harm-benefit analysis.
Joseph Fraiman, Juan Erviti, Mark Jones, Sander Greenland, Patrick Whelan, Robert M. Kaplan, Peter Doshi, Serious adverse events of special interest following mRNA COVID-19 vaccination in randomised trials in adults, Vaccine, 2022, ISSN 0264-410X, https://doi.org/10.1016/j.vaccine.2022.08.036.
Do NOT comply.


what you get when you do your cost/benefit analysis after the fact. love peter doshi
I was in the third grade during Regans 2nd term and I learned then to Just say no to drugs!